Retesting Through Updated Genetic Testing is Useful Across a Range of Hereditary Cancer Syndromes
This research is from the Michigan Department of Health and Human Services and the Karmanos Cancer Institute.
Genetic testing for hereditary cancer syndromes has evolved rapidly leading to the discovery of many cancer predisposition genes. After the Supreme Court’s ruling in the case of The Association for Molecular pathology v. Myriad in June 2013, the availability of genetic testing and multigene panels widely expanded [1]. Since then, multigene panels have been shown to save time, be cost effective, and increase diagnostic yield [2]. Retesting patients who were initially tested by a phenotype-directed method has also been shown to increase diagnostic yield. However, since most payers have historically covered only one round of BRCA1/2 genetic testing for eligible patients, most investigations have focused specifically on initial testing of BRCA1/2 [3]. Only a few studies have looked at retesting outcomes when first line testing included more genes [4]. As a result, further evidence is needed to determine the utility of retesting outside of breast and ovarian cancer populations and to identify which patients to prioritize for updated testing.
Study and Methods
To determine the outcomes of retesting and identify predictors associated with positive findings upon retesting, Dettwyler et al recently conducted a retrospective chart review for patients at a cancer genetics clinic between January 1998 – March 2019 [5]. Patients were eligible if they had germline genetic testing on ≥2 dates with ≥1 calendar year between any two rounds of genetic testing. Additionally, they must have not had a pathogenic variant (PV) or likely pathogenic variant (LPV) on first line genetic testing.
Overall Results
A total of 6556 patients were identified on the registry and 139 had multiple rounds of genetic testing that met study inclusion criteria. Of the 139 patients, 104 patients were affected with a cancer/tumor warranting genetic testing and 35 were unaffected. Both sub-cohorts were predominantly female and non-Hispanic White. Upon retesting, 24 patients were identified to have a PV/LPV, 21 of these patients were affected patients. Fourteen patients total were found to have a variant of uncertain significance (VUS) upon retesting.
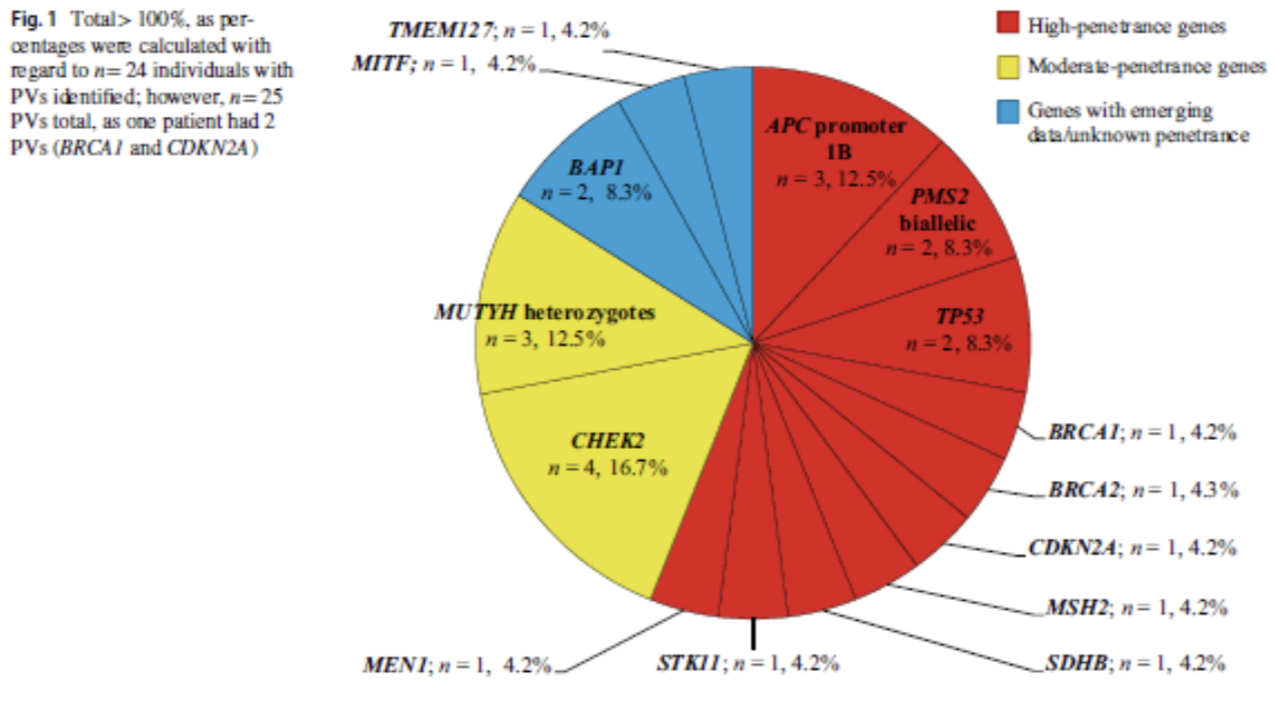
As reflected in the figure below, a total of 25 PV/LPVs were identified with 14 syndromes. 13 of these variants were identified in high penetrance genes and 7 were found in moderate or low penetrance genes. The remaining 4 genes were in recently described genes with emerging cancer risk data.
Results by initial indication:
For those initially referred for BRCA1/2 genetic testing (n=57), 9 were found to have a PV/LPV. For those initially referred for Lynch Syndrome testing (n=20), 3 were found to have a PV/LPV. For those initially referred for other indications (n=63), 13 had PV/LPVs. Overall, they were largely clinically consistent with the patient’s personal or family history of cancer but discordant with the initial phenotype-directed reason for referral.
Reason for returning to testing:
Although there could be multiple reasons for patients returning for genetic testing, the most common was a new diagnosis of cancer or tumor (60%). Other reasons were changes in their history (41% of affected patients and 42% of unaffected patients), and updated testing (45% of affected patients and 57% of unaffected patients).
Clinical impact:
The authors’ exploration of the clinical impact of retesting showed significant changes in management recommendations for 66% of those identified to have PV/LPV.
Overall, this study found that retesting identified PV/LPVs in approximately 1 out of every 6 individuals with previously uninformative genetic testing, regardless of first-line genetic testing indication.
Retesting patients based on updated genetic testing availability could result in earlier cancer syndrome identification, significantly impacting screening recommendations and potentially reducing cancer-related morbidity and mortality. Furthermore, this research suggests that affected individuals might be best to prioritize when thinking about who to retest as most of the PV/LPV were found in affected patients.
Should you have any questions or would like further information, please contact Nancie Petrucelli at (313) 576-8704/[email protected].
References:
Offit K, Bradbury A, Storm C et al (2013) Gene patents and personalized cancer care: impact of the Myriad case on clinical oncology. J Clin Oncol 31:2743–2748.
Kurian AW, Hare EE, Mills MA et al (2014) Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol 32:2001–2009.
3. Clain E, Trosman JR, Douglas MP et al (2015) Availability and payer coverage of BRCA1/2 tests and gene panels. Nat Biotechnol 33:900–902.
4. Frey MK, Kim SH, Bassett RY et al (2015) Rescreening for genetic mutations using multi-gene panel testing in patients who previously underwent non-informative genetic screening. Gynecol Oncol 139:211–215.
5. Dettwyler SA, Koeppe ES, Jacobs MF, Stoffel EM (2022). Outcomes of retesting in patients with previously uninformative cancer genetics evaluations. Fam Cancer 21(3):375-385.
back to blog